Enfermedad pulmonar intersticial asociada a esclerosis sistémica (EPI-ES)
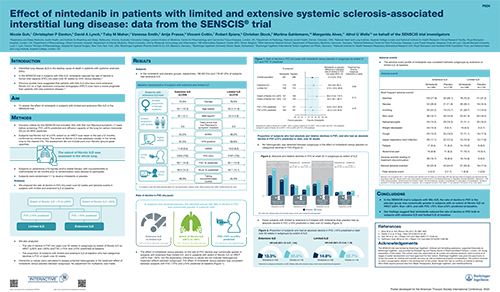
Effect of nintedanib in patients with limited and extensive systemic sclerosis-associated interstitial lung disease (SSc-ILD): data from the SENSCIS trial
Goh N et al.
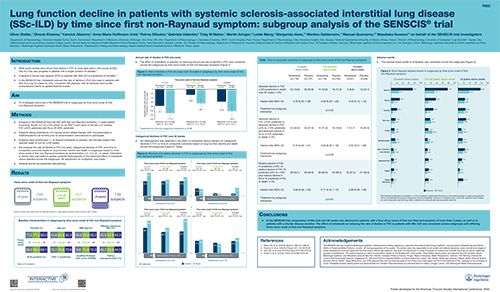
Lung function decline in patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD) by time since first non-Raynaud symptom: subgroup analysis of the SENSCIS trial
Distler O et al.
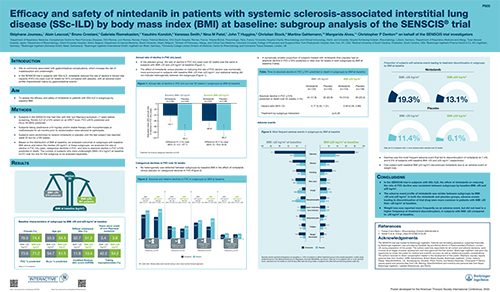
Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD) by body mass index (BMI) at baseline: subgroup analysis of the SENSCIS trial
Jouneau S et al.
Effect of nintedanib on progression of systemic sclerosis-associated interstitial lung disease (SSc-ILD) beyond 52 weeks: data from the SENSCIS trial
Maher TM et al.